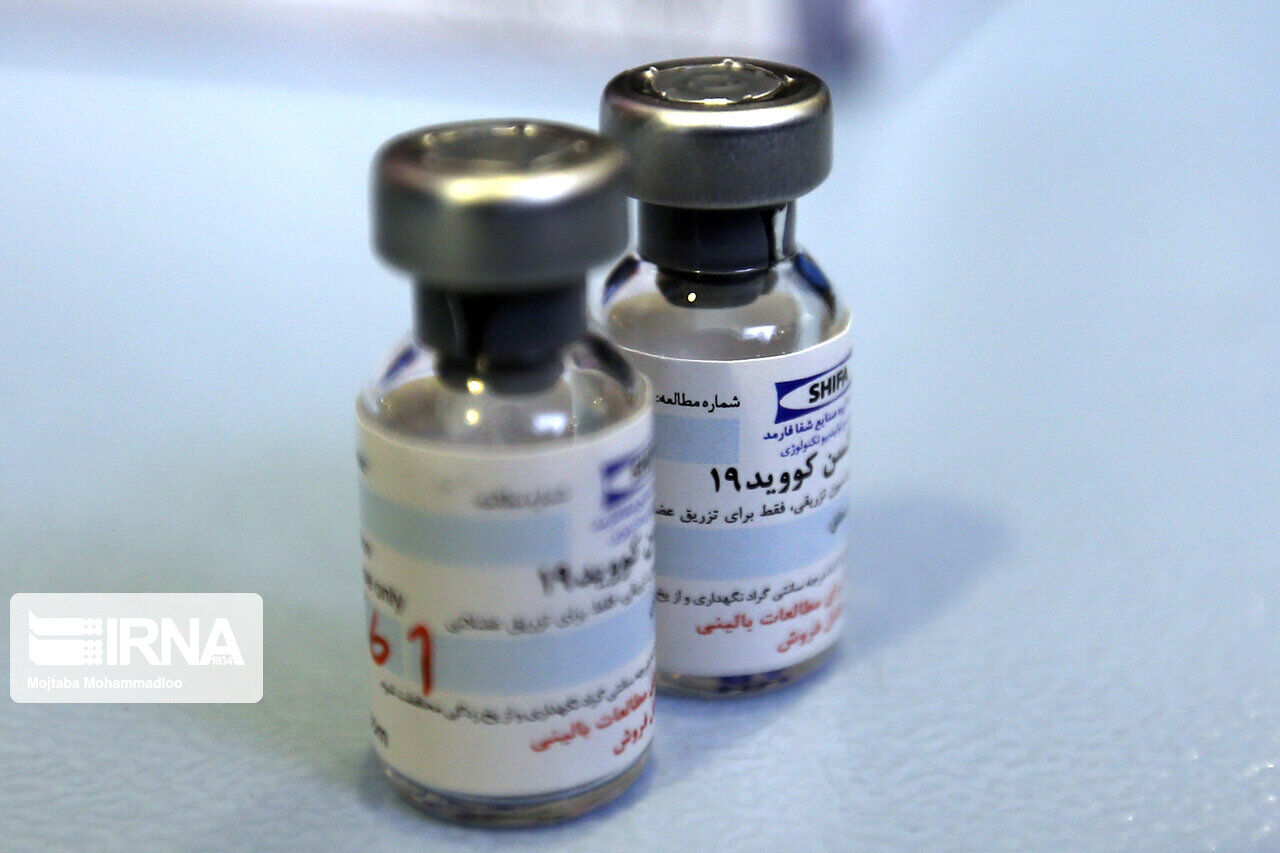
Salehi said that Shafa Pharmed Company and Barakat Complex are reviewing the requests of some foreign countries to enter the third phase of the clinical trial of this vaccine.
In case of contracting and successful result of this vaccine in the third stage of human testing, these countries can also use this vaccine in their public vaccination, he added.
Phases 2 and 3 of the clinical trial for the first Iranian coronavirus vaccine, COVIran Barakat, began on Monday with two voluntary receivers.
The first phase of the COVIran Barakat test was carried out early in January and 56 volunteers at the age of 18-50 received two doses of the vaccine.
The collection of blood samples from phase one volunteers was completed recently and the final report was handed over to Iran's Health Ministry.
3266**2050
Follow us on Twitter @IrnaEnglish
IRNA English solhkhabar | Peace International News Agency Peace International News Agency , Peace News , International Agency News of Peace
solhkhabar | Peace International News Agency Peace International News Agency , Peace News , International Agency News of Peace